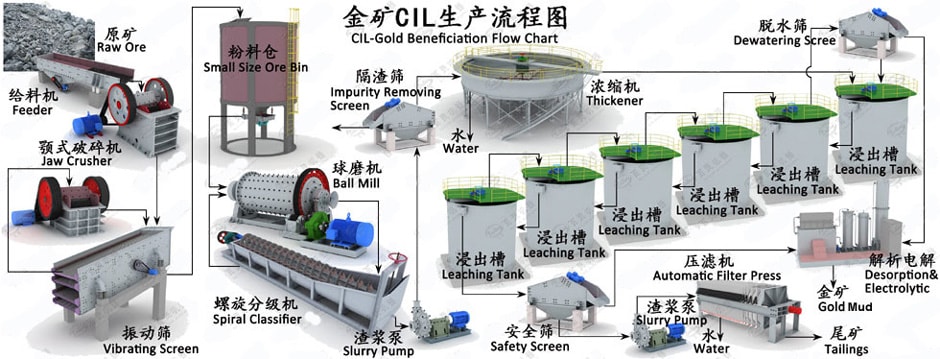
Cyanide leaching is the standard method used for recovering most of the gold
throughout the world today. The process originated around 1890 and quickly
replaced all competing technologies. The reason was strictly economical in
nature. Where amalgamation plants could recover about 60% of the gold present,
cyanide could recover about 90%. Because of the improved recovery, many of
the old tailings piles from other processes have been economically reprocessed
by cyanide leaching. Cyanide is as close to a “universal solvent” for gold
as has been developed. Other leaching reagents will only work on very specific
types of ore.
The standard cyanide leach process consists of grinding the ore to about
80% – 200 mesh, mixing the ore/water grinding slurry with about 2 pounds
per ton of sodium cyanide and enough quick lime to keep the pH of the solution
at about 11.0. At a slurry concentration of 50% solids, the slurry passes
through a series of agitated mixing tanks with a residence time of 24 hours.
The gold bearing liquid is then separated from the leached solids in thickener
tanks or vacuum filters, and the tailings are washed to remove gold and cyanide
prior to disposal. The separation and washing take place in a series of units
by a process referred to as counter current decantation (CCD). Gold is then
recovered from the pregnant solution by zinc precipitation and the solution
is recycled for reuse in leaching and grinding.
CARBON ADSORPTION RECOVERY
Granular coconut shell activated carbon, is widely used for recovery of gold
from cyanide solutions. The process can be applied to clean solutions through
fluidized bed adsorption columns, or directly to leached ore slurries by
the addition of carbon to agitated slurry tanks, followed by separation of
the carbon from the slurry by coarse screening methods.
Gold cyanide is adsorbed into the pores of activated carbon, resulting in
a process solution that is devoid of gold. The loaded carbon is heated by
a strong solution of hot caustic and cyanide to reverse the adsorption process
and strip the carbon of gold. Gold is then removed from the solution by
electrowinning. Stripped carbon is returned to adsorption for reuse.
The major advantage of carbon-in-pulp recovery over Merrill Crowe recovery
is the elimination of the leached ore solids and liquid separation unit
operation. The separation step typically involves a series of expensive gravity
separation thickeners or continuous filters arranged for countercurrent washing
or filtration of the solids. For ores exhibiting slow settling or filtration
rates, such as ores with high clay content, the countercurrent decantation
(CCD) step can become cost prohibitive.
Ores with high silver content will generally suggest that Merrill-Crowe recovery
be used. This is because of the very large carbon stripping and electrowinning
systems required for processing large quantities of silver. The typical rule
of thumb states that economic silver to gold ratios of greater than 4 to
1, will favor installation of a Merrill-Crowe system, but this decision can
be altered if the ore exhibits very slow settling rates.
There are several variations to the carbon adsorption process including:
- Carbon-In-Column (CIC): With carbon-in-column operation, solution flows
through a series of fluidized bed columns in an upflow direction. Columns
are most frequently open topped, but closed top pressurized columns are
occasionally used.
Carbon columns are most commonly used to recover gold and silver from heap
leach solutions. The major advantage of fluidized bed carbon columns is their
ability to process solutions that contain as much as 2 to 3 wt% solids. Heap
leach solutions are frequently high in solids due to fine particle washing
from heaps. Down flow carbon columns are rarely used for gold recovery, because
they act like sand filters and are subsequently subject to frequent plugging.
- Carbon-In-Pulp (CIP): Carbon-in-pulp operation is a variation of the
conventional cyanidation process. Ore is crushed, finely ground, and cyanide
leached in a series of agitated tanks to solubilize the gold values. Instead
of separating solids from the pregnant solution, as in the traditional
cyanidation process, granular activated carbon is added to the leached slurry.
The carbon adsorbs the gold from the slurry solution and is removed from
the slurry by coarse screening. In practice, this is accomplished by a series
of five or six agitated tanks where carbon and ore slurry are contacted in
a staged countercurrent manner.
This greatly increases the possible gold loading onto the carbon while
maintaining a high recovery percentage. Carbon is retained within the individual
CIP tanks by CIP tank screens. The opening size of the CIP tank screens is
such that the finely ground ore particles will pass through the screens,
but the coarse carbon will not. Almost every imaginable type of screen has
been tried for this application, with some types being much more successful
than the rest.
- Carbon-In-Leach (CIL): The carbon-in-leach process integrates leaching
and carbon-in-pulp into a single unit process operation. Leach tanks are
fitted with carbon retention screens and the CIP tanks are eliminated. Carbon
is added in leach so that the gold is adsorbed onto carbon almost as soon
as it is dissolved by the cyanide solution. The CIL process is frequently
used when native carbon is present in the gold ore. This native carbon will
adsorb the leached gold and prevent its recovery. This phenomenon is referred
to commonly as “preg-robbing”. The carbon added in CIL is more active than
native carbon, so the gold will be preferentially adsorbed by carbon that
can be recovered for stripping. The CIL process will frequently be used in
small cyanide mills to reduce the complexity and cost of the circuit.
There are several disadvantages to CIL compared with CIP. Carbon loading
will be 20 to 30% less than with CIP, which means more carbon has to be stripped.
(This disadvantage may be overcome by a hybrid circuit, incorporating a cross
between CIL and CIP.) The CIL process requires a larger carbon inventory
in the circuit, which results in a larger in-process tie up of gold. The
larger carbon inventory can also result in higher carbon (and gold) losses
through carbon attrition.